Ionic equations are chemical equations in which electrolytes in aqueous solutions are expressed as dissociated ions, similar to a molecular equation. To indicate that the ions are in an aqueous solution, the equation usually includes (aq) to indicate that they are dissolved in water.
Ion-dipole interactions with water molecules stabilize ions in aqueous solutions. An ionic equation can, however, be written for any electrolyte that dissociates and reacts in a polar solvent. Both sides of the reaction arrow contain the same type and number of atoms in a balanced ionic equation. In addition, both sides of the equation have the same net charge.
In aqueous solution, strong acids, strong bases, and soluble ionic compounds (usually salts) exist as dissociated ions, thus they are written as ions. Only a small amount of weak acids and bases and insoluble salts dissociate into ions, so they are usually written using their molecular formulas. When it comes to acid-base reactions, there are exceptions.
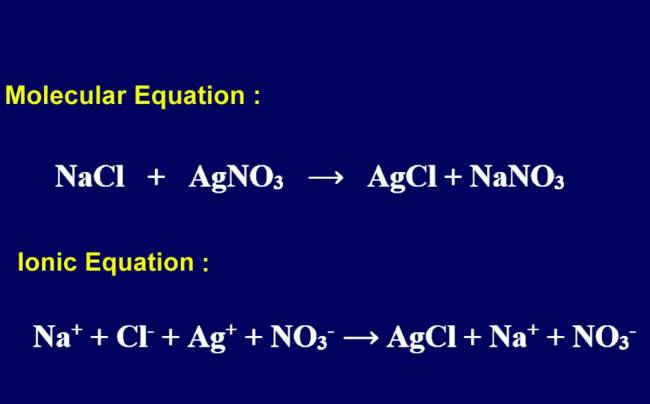
Table of Contents
Examples of Ionic Equations
Ag+(aq) + NO3-(aq) + Na+(aq) + Cl-(aq) → AgCl(s) + Na+(aq) + NO3-(aq) The following ionic equation describes the reaction:
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
Complete Versus Net Ionic Equation
Completable ionic equations and net ionic equations are the two most common types of ionic equations. A complete ionic equation indicates all of the dissociated ions in a chemical reaction. Due to the fact that they don’t participate in the reaction of interest, the net ionic equation cancels out ions that appear on both sides of the reaction arrow. A spectator ion is an ion that cancels out an ion.
In the reaction between silver nitrate (AgNO3) and sodium chloride (NaCl) in water, the complete ionic equation is:
Ag+(aq) + NO3-(aq) + Na+(aq) + Cl-(aq) → AgCl(s) + Na+(aq) + NO3-(aq)
Notice that both the sodium cation Na+ and the nitrate anion NO3- are present on both the reactants and products sides of the arrow. The net ionic equation can be written as follows if they are canceled out:
Ag+(aq) + Cl-(aq) → AgCl(s)
Each species in this example had a coefficient of 1 (which is not written). For example, if every species started with 2, each coefficient would be divided by a common divisor to obtain the net ionic equation.
Balanced equations should be written for both the complete ionic equation and the net ionic equation.
